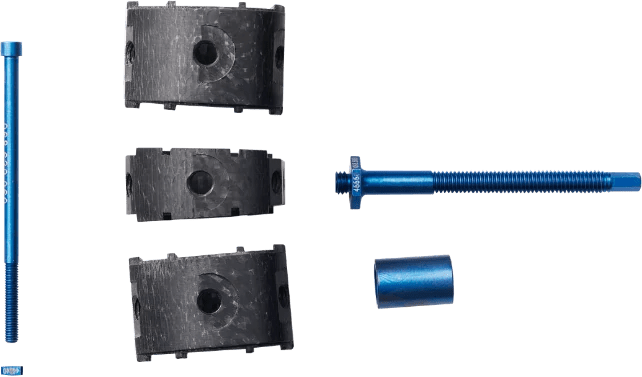
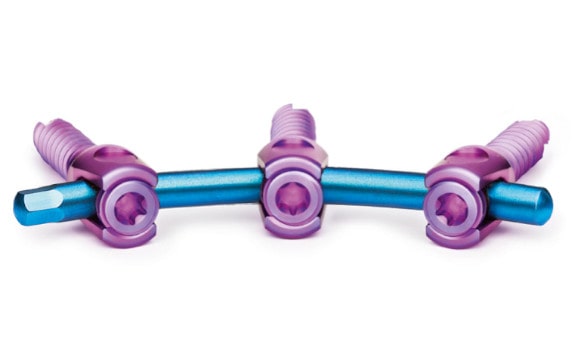
Carbon composite technology for spine fusion.
ostaPek® implants introduce high-performance long fiber carbon composite material to spine fusion. Based on fiber pattern design specific to each indication, surgeons and patients can now consider treatment options not available based on traditional methods and materials.
Learn more about ostaPek®