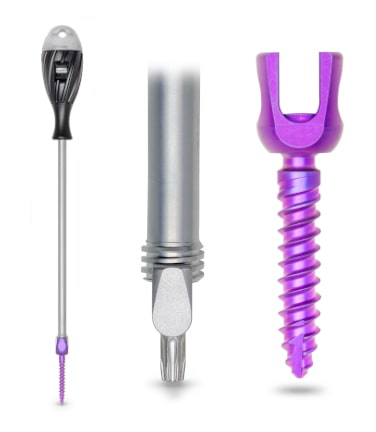
Spine Nuances is excited to announce that the GII pedicle fixation system now includes the next generation flexStaas pedicle screw that was recently cleared by the FDA for the US market.
The flexStaas pedicle screw as part of the Coligne GII spinal fixation system is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion. The poly-axial flexStaas pedicle screw features (1) a tulip design with a large docking surface and click grip for a fast and strong connection, (2) double helix cancellous thread with a self-tapping tip for fast and precise screw placement and (3) an increased resistance to torque. The set screw has (1) a wide self-aligning thread that facilitates set screw positioning and tightening and (2) a torx interface to increase axial compression for Coligne Titanium rods.
The flexStaas pedicle screw part of the Coligne GII Spinal Fixation System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion. It is intended for use in the non-cervical posterior spine, in skeletally-mature patients, as an adjunct to fusion using autograft and/or allograft. The GII Spinal Fixation System is intended for use for one or more of the following: (1) degenerative disc disease (defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies), (2) degenerative spondylolisthesis with objective evidence of neurologic impairment, (3) fracture, (4) dislocation, (5) spinal stenosis, (6) deformities or curvatures (i.e. scoliosis, kyphosis, and/or lordosis), (7) spinal tumor, and/or (8) failed previous fusion (pseudoarthrosis).
When used for posterior non-cervical pedicle screw fixation in pediatric patients, the Coligne GII Spinal Fixation System is indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. Additionally, the system is intended to treat pediatric patients diagnosed with the following conditions: spondylolisthesis/spondylolysis and fracture caused by tumor and/or trauma.
The system is intended to be used with autograft and/or allograft. Pediatric pedicle screw fixation is limited to a posterior approach.
9 June 2021